Catalytic Amidation Reactions
News
- Tom gave an online talk on "Organocatalytic Amide Formation: From Pharmaceutical Synthesis to Prebiotic Chemistry" at the Merck/Sigma-Aldrich Chemistry Minds event on 20th October.
Welcome to the website for the EPSRC-funded project Development of a generally applicable catalytic amidation reaction which aims to develop and promote the use of catalytic amidation reactions between amines and carboxylic acids. This project is a collaboration between the research groups of Tom Sheppard (University College London), Andy Whiting (University of Durham), Henry Rzepa (Imperial College London), and Jordi Burés (University of Manchester) to develop a detailed mechanistic understanding of a variety of catalytic amidation reactions and use this understanding to design new and improved catalysts. We are pleased to be working with project partners in industry to promote the wider use of catalytic amidation in industrial applications. The project began in January 2021.
The amide unit is one of the most prevalent functional groups in organic molecules, and amide formation is one of the most common reactions in organic chemistry. Most commonly, this involves the reaction of a carboxylic acid and an amine using a stoichiometric activating agent, which formally promotes water removal to yield the amide, e.g. chlorinating agent such as SOCl2 or PCl5, coupling reagent such as EDC, T3P, PyBOP, HATU, etc. All of these approaches are highly inefficient, however, as they generate stoichiometric quantities of waste products that are often hazardous to dispose of. A catalytic amide formation between a carboxylic acid and an amine is therefore of great interest as the only stoichiometric byproduct would be water. Whilst there have been many reports of catalytic amidation reactions to date, they are rarely used by most synthetic chemists on a day-to-day basis. Partly this is a result of the limited scope of many of these reactions, but it is likely down to a lack of familiarity with catalytic amidation reactions and how they can be applied. Our project aims to address this issue by developing new and improved methods of achieving catalytic amidation reactions, but also by promoting the wider use of catalytic amidation reactions in synthetic chemistry.
This aim of this website is to provide useful information and resources for anyone wanting to perform a catalytic amidation reaction, as well as information about the project team and the research project outcomes. Below is an overview of our recent work which underpins this EPSRC-funded research project and you can find details of the research project team here. You will find an ever-growing summary of the many catalytic amidation reactions reported in the literature, as well as a user guide to help you use a catalytic amidation reaction in your own research including information on how to select a catalyst, reaction conditions, etc to maximise the chances of a successful reaction with your substrates. Whilst this project focuses primarily on catalytic direct amidation reactions of amines and carboxylic acids, as this represents the most common approach which is able to exploit the huge array of commercially available building blocks containing these functional groups, alternative catalytic approaches to amides have also been reported to prepare amides from esters, alcohols, aldehydes, aryl halides, etc.
Updates on research outputs from this project will be added to this page as the project progresses.
Publications from this project
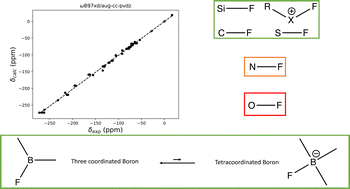
3. A. S. Dumon, H. S. Rzepa, C. Alamillo-Ferrer, J. Bures, R. Procter, T. D. Sheppard, A. Whiting, A computational tool to accurately and quickly predict 19F NMR chemical shifts of molecules with fluorine-carbon and fluorine-boron bonds, Physical Chemistry & Chemical Physics, 2022, 24, 20409. doi: 10.1039/D2CP02317B Preprint
Publications from previous collaborative work
2. H. R. Rzepa, S. Arkhipenko, E. Wan, M. T. Sabatini, V. Karaluka, A. Whiting, T. D. Sheppard, An Accessible Method for DFT Calculation of 11B NMR Shifts of Organoboron Compounds, The Journal of Organic Chemistry 2018, 83, 8020. doi: 10.1021/acs.joc.8b00859
This paper describes a simple DFT method developed by Henry Rzepa for the straightforward calculation of 11B NMR shifts. We have found this approach to be very useful for the identification of possible reaction intermediates in often complex mixtures.
1. S. Arkhipenko, M. Sabatini, A. Batsanov, V. Karaluka, T. D. Sheppard, H. Rzepa. A. Whiting, Mechanistic insights into boron-catalysed direct amidation reactions, Chemical Science 2018, 9, 1058. Open Access. doi: 10.1039/C7SC03595K
Highlighted by Prof Andrei Yudin in his editor's choice article collection of important recent papers in the journal.
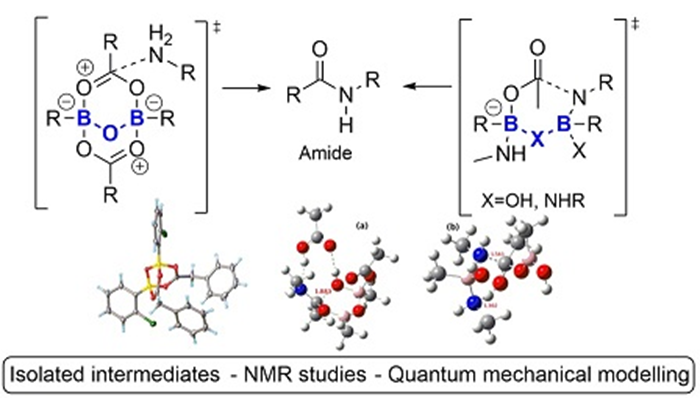
This paper describes our collaborative work on boronic acid-catalysed amidation reactions in which we demonstrated that activation of a carboxylic acid for amidation likely involves the concerted action of at least two boron atoms via formation of bridged B-X-B units. Detailed studies of the interaction of both carboxylic acids and amines with a variety of catalysts provided insights into the species that could readily be formed in amidation reaction mixtures. Pathways involving B-X-B dimers for carboxylic acid activation were found via DFT calculations to be considerably lower in energy than the previously proposed acyloxyboronate mechanism and they are thus much more consistent with reactions at ambient temperature.
Tel: +44 (0)20 7679 2467 Email: tom.sheppard@ucl.ac.uk
