Catalytic Amidation Methods
Many different catalytic methods have been reported in the literature for the direct amidation reaction between amines and carboxylic acids. Below is a growing list of the available methods which we will continue to update. You will find details of the catalysts (including links to commercial suppliers, if available), reaction solvents/conditions, an overview of the reaction scope based on published information, and links to the scientific literature reports in which these catalysts were reported. Reactions which require the use of any stoichiometric activating agent are not included, with the exception of dehydrating agents such as molecular sieves.
Boron Catalysts
B(OCH2CF3)3
Smiles: FC(COB(OCC(F)(F)F)OCC(F)(F)F)(F)F
InChI Key: DIEXQJFSUBBIRP-UHFFFAOYSA-N
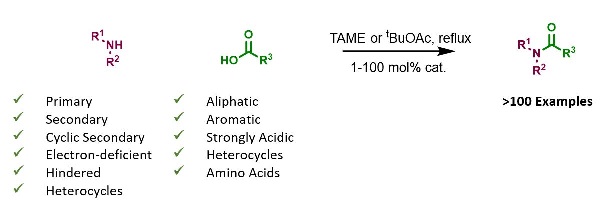
References
Borate esters: Simple catalysts for the sustainable synthesis of complex amides doi: 10.1126/sciadv.1701028
Protecting group free amidation of amino acids using Lewis acid catalysts doi: 10.1002/chem.201800372
Catalytic Direct Amidations in tert-Butyl Acetate Using B(OCH2CF3)3 doi: 10.1039/C9OB01012B
Suppliers
Boric Acid
Smiles: OB(O)O
InChI Key: 1/BH3O3/c2-1(3)4/h2-4H
 References
References
Boric Acid Catalyzed Amide Formation from Carboxylic Acids and Amines: n-Benzyl-4-phenylbutyramide doi: 10.15227/orgsyn.081.0262
Discussion Addendum for: Boric Acid Catalyzed Amide Formation from Carboxylic Acids and Amines: n-Benzyl-4-phenylbutyramide doi: 10.15227/orgsyn.089.0432
Suppliers5-Methoxy-2-iodophenylboronic acid (MIBA)
Smiles: OB(O)C1=C(I)C=CC(OC)=C1
InChI Key: XQYAEIDOJUNIGY-UHFFFAOYSA-N
 References
References
Direct Amidation of Carboxylic Acids Catalyzed by ortho-Iodo Arylboronic Acids: Catalyst Optimization, Scope, and Preliminary Mechanistic Study Supporting a Peculiar Halogen Acceleration Effect doi: 10.1021/jo3013258
Direct and Waste-Free Amidations and Cycloadditions by Organocatalytic Activation of Carboxylic Acids at Room Temperaturedoi: 10.1002/anie.200705468
A multigram-scale lower E-factor procedure for MIBA-catalyzed direct amidation and its application to the coupling of alpha and beta aminoacidsdoi: 10.1039/C5GC00659G
SuppliersN,N-di-isopropylbenzylamineboronic acid
Smiles: OB(O)C1=C(I)C=CC(OC)=C1
InChI Key: XQYAEIDOJUNIGY-UHFFFAOYSA-N
 References
References
Synthesis, evaluation and application of novel bifunctional N,N-di-isopropylbenzylamineboronic acid catalysts for direct amide formation between carboxylic acids and amines doi: 10.1039/B712008G
Suppliers’pym-DATB’
Smiles: B1(C2=CC=CC=C2C3=C(N1)C(=NC=N3)C4=CC=CC=C4B5OB6C7=CC=CC=C7C8=C9N6B(O5)C1=CC=CC=C1C9=NC=N8)O
InChI Key: UJSSLUDYAHIVLK-UHFFFAOYSA-N
 References
References
Unique physicochemical and catalytic properties dictated by the B3NO2 ring systemdoi: 10.1038/nchem.2708
All Non-Carbon B3NO2 Exotic Heterocycles: Synthesis, Dynamics, and Catalysis doi: 10.1002/chem.201900715
Neighboring Protonation Unveils Lewis Acidity in the B3NO2 Heterocycledoi: 10.1021/jacs.8b10336
Suppliers
Metal Catalysts
Titanium(IV) isopropoxide
Smiles: CC(C)O[Ti](OC(C)C)(OC(C)C)OC(C)C
InChI Key: VXUYXOFXAQZZMF-UHFFFAOYSA-N
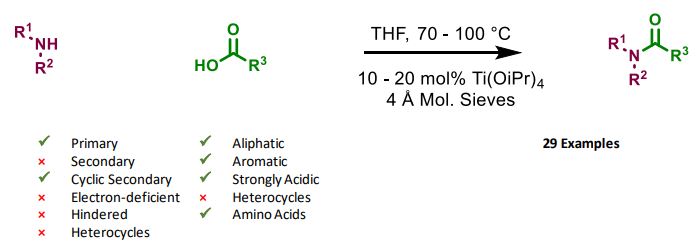 References
References
Titanium(IV) Isopropoxide as an Efficient Catalyst for Direct Amidation of Nonactivated Carboxylic Acids doi: 10.1055/s-0032-1316993
Protecting-Group-Free Amidation of Amino Acids using Lewis Acid Catalysts doi: 10.1002/chem.201800372
SuppliersZirconocene dichloride (Bis(cyclopentadienyl)zirconium(IV) dichloride)
Smiles: [cH-]1cccc1.[cH-]1cccc1.[Cl-].[Cl-].[Zr+4]
InChI Key: QMBQEXOLIRBNPN-NUQVWONBAX
 References
References
Direct amide formation from unactivated carboxylic acids and amines doi: 10.1039/c1cc15210f
Zirconium catalyzed amide formation without waterscavenging doi: 10.1002/aoc.5062
Suppliers Tel: +44 (0)20 7679 2467 Email: tom.sheppard@ucl.ac.uk